PDF chapter test TRY NOW
When an acid and a base are combined, a chemical reaction occurs. This process is called neutralisation. The reaction mixture produces heat during neutralisation; hence, it is an exothermic reaction.
In neutralisation reaction, a new substance is formed, which is known as salt. The salt produced here can be either acidic, basic or neutral.
The reaction between the hydrochloric acid and sodium hydroxide gives salt and water as a product. It is one example for neutralization reaction.
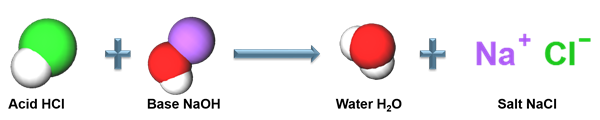
Acid-base reaction
Similarly, other acids also produce salt and water when reacting with the base. Let us see some examples of the reaction of acids with sodium hydroxide (\(NaOH\)).
Reference:
https://ecampusontario.pressbooks.pub/app/uploads/sites/372/2019/01/acid_base_reaction.png
