PDF chapter test TRY NOW
We know that iron undergoes rusting with oxygen and forms iron oxide. This process can be used to estimate the percentage of oxygen in air, which has been removed by the rusting reaction.
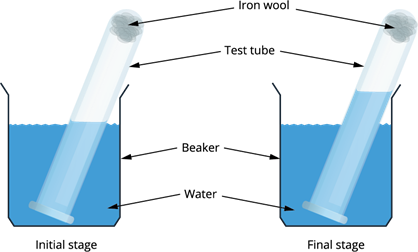
Experimental set-up
- Take a small portion of iron wool, press it into a \(20\ ml\) graduated test tube and wet it with water.
- Tip away excess of water.
- Take a \(500\ ml\) beaker and fill half of the beaker with water.
- Invert the test tube and place it in air.
- Leave the arrangement at least for a week without making any disturbance to the test tube.
- Observe the changes that had happened in the iron wool and to the level of water inside the test tube.
Observation:
We could see that the water level has inside the test tube. The rise in water is because of in air which has been removed by the reaction.
This will be about which is approximately the percentage of in the air.
