PDF chapter test TRY NOW
The density of a particular substance, under specific conditions, remains the same. Therefore, the density of a substance is one of its characteristic properties. It is different for different substances.
For example, the density of gold is 19300 , while that of water is \(1000\) . The density of a given sample of a material can help us to determine its purity. It is easy to express the density of a substance in comparison with that of water.
For example, the density of gold is 19300 , while that of water is \(1000\) . The density of a given sample of a material can help us to determine its purity. It is easy to express the density of a substance in comparison with that of water.
Relative density
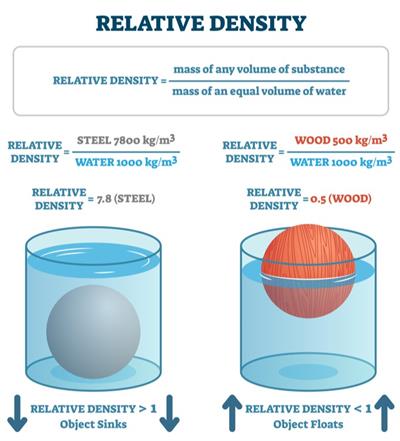
The relative density of a substance is the ratio of its density to that of water.
The relative density has similar quantities in numerator and denominator. Therefore, it has no unit.
Example:
1. The density of water is . Find the density of silver, whose relative density is \(10.8\).
Given data:
Relative density of gold \(=\) \(10.8\)
Density of water \(=\)
To find: Density of the material
We know,
Apply the known values,
