
PUMPA - SMART LEARNING
எங்கள் ஆசிரியர்களுடன் 1-ஆன்-1 ஆலோசனை நேரத்தைப் பெறுங்கள். டாப்பர் ஆவதற்கு நாங்கள் பயிற்சி அளிப்போம்
Book Free DemoThe solubility of a solute is the sum of the solute present in a saturated solution at this temperature.
The Concentration of a Solution:
A solution's concentration measures how much solute has dissolved in a given volume of solvent or solution. The concentrated solution is one in which the volume of dissolved solute is relatively high.
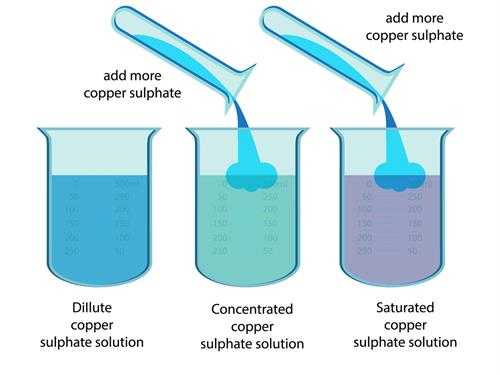
Concentration of the solution in the image increases from left to right
The formula below is used to calculate the concentration of a solution.
| \(\text{Concentration of solution}\) \(=\) \(\frac{\text{Amount of solute}} {\text{Amount of solvent or solution}}\) |
There are various ways to express the concentration of a solution, but here, we will learn these three well-known methods:
- Mass by mass percentage
- Mass by volume percentage
- Volume by volume percentage
1. Mass by mass percentage.
We can find the mass percentage of a solution by dividing the mass of solute by the mass of the solution and multiply the product by \(100\).
| Mass by mass percentage \(=\) \(\frac{\text{Mass of solute}}{\text{Mass of solution}}\) \(\times100\) |
2. Mass by volume percentage.
We can find the mass volume percentage of a solution by dividing the mass of solute by the volume of solution and multiply the product by \(100\).
| Mass by volume percentage \(=\) \(\frac{\text{Mass of solute}}{\text{Volume of solution}}\)\(\times100\) |
3. Volume by volume percentage.
We can find the volume percentage of a solution by dividing the volume of solute by the volume of solution and multiply the product by \(100\).
Volume by volume percentage \(=\) \(\frac{\text{Volume of solute}}{\text{Volume of solution}}\)\(\times100\) |